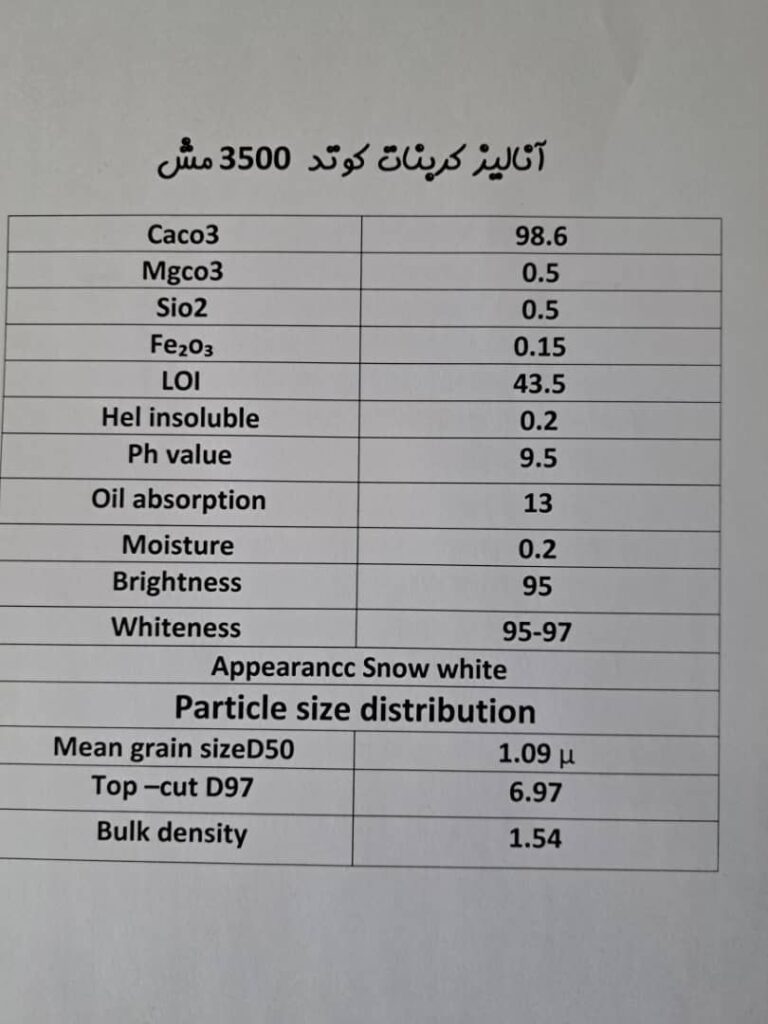
Calcium carbonate is a mineral substance with the formula CaCO3, which is one of the most widely used substances known to mankind, this substance can be produced artificially, and of course, it is also present in eggshells and the membranes of animals such as snails and crabs. Pearls are also made of calcium carbonate.
Applications of calcium carbonate:
Calcium carbonate is extracted from the mine for industrial use. Pure calcium carbonate can be produced from marble or it can be formed by passing carbon dioxide through a calcium hydroxide solution.
Calcium carbonate is converted into lime by heat with the loss of CO2, which is used in construction works, in the iron mining industry and steel production as a smelting aid and for the preparation of calcium carbide. Lime is also used in agriculture to improve soil and adjust its pH.
Calcium carbonate is used in tile and ceramic industries, especially in the glazing industry. Due to the eutectic formation, it lowers the melting point of the set and when used in glaze, it lowers the thermal expansion coefficient of the glaze.
Calcium carbonate is one of the most widely used minerals, which is used in the paper, plastics, paints and coatings industries both as a filler and as a color coating (due to its special white color).
In the paper industry, it is valued all over the world for its high brightness and light scattering, and it is used as an inexpensive filler to brighten dull paper surfaces.
Manufacturing materials and construction.
calcium carbonate bulk price:
to receive calcium carbonate bulk price, please contact to us.
calcium carbonate producers:
In Iran there are nearly 20 calcium carbonate producers, its produced in different mesh (size) and also different coatings.
precipitated calcium carbonate manufacturers:
this type of calcium carbonate is chemically treated which also can be supplied from Iran.
Precipitated calcium carbonate manufacturers in Iran are not many but it can be supplied without any problems.
where to get calcium carbonate?
To get calcium carbonate, you can contact to us.