Barium carbonate is one of the most widely used chemicals in the industry, which exists both naturally in mines and is produced industrially. This compound has the chemical formula BaCO3. Another name for barium carbonate is witrite. Barium carbonate is physically solid and does not dissolve in water. This substance has a crystalline structure whose color is mainly white or pale yellow. Barium carbonate is sensitive to heat and when we heat it, it turns into barium oxide and carbon dioxide.
Application of barium carbonate
Ceramic production
Barium carbonate reacts well with coloring oxides and causes beautiful colors to be produced for the production of ceramics. Barium carbonate can also be used in the composition of tiles produced from clay to create various colors.
Brick production
In brick making, barium carbonate is mixed with clay to convert soluble sulfates into insoluble sulfates. The presence of these soluble sulfates causes the ceramics to not have a uniform surface and to have low and high, and as a result, they lose their beauty.
Production of barium oxide
Barium oxide is obtained from barium carbonate. In many industries, barium oxide is used instead of lead or hydrated lime. That is why they also use it in making glass. Also, in glazing, a certain amount of barium oxide is used, which can make the glazes shiny.
Production of electronics
The applications of barium carbonate for making electronic components, capacitors, etc. are also wide
Barium carbonate in drilling oil wells
This chemical is used to prevent coagulation in drilling oil wells.
Application in the production of television image lamp cells
Barium carbonate has a good cathodic potential. For this reason, cathode ray tubes are used in televisions to improve the quality of television images and make their colors more transparent.
Absorption of X-rays and harmful radiation
This material can absorb X-rays well and prevent the spread of these rays in different environments, especially in the imaging environments of medical devices.
Production of agricultural pesticides
Since this chemical compound has toxic properties, it is used in the production of many pesticides for agricultural products, rodent poisons such as mice, insecticides, germicides, etc.
Glass manufacturing industry
In the production of some types of glass, especially medical glasses, this material is used for greater transparency and increased resistance of the glass. It should be mentioned that lead oxide is used in the production of various glasses. The glasses that use lead oxide in their production are heavier and harder than the glasses that are made of barium oxide, but their brightness is the same and they are not different from each other.
Other applications
Among other uses of this chemical, we can mention the production of photographic papers, titanates, welding, etc.
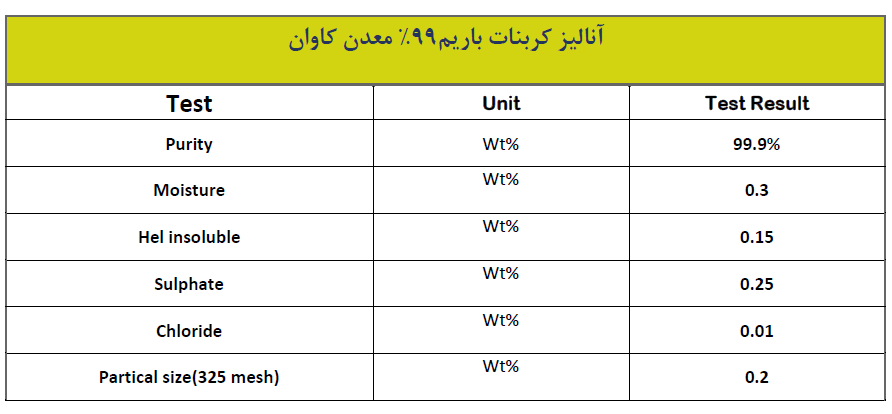
You can see analysis of one of the Barium carbonate produced in Iran.